Abstract
Introduction:
Chimeric antigen receptor (CAR) modified T cells targeting B-cell maturation antigen (BCMA) have shown activity in a case series of patients with relapsed or refractory Multiple Myeloma (MM), but feasibility, toxicity, and response rates of consecutively enrolled patients treated with a consistent regimen and assessed on an intention-to-treat basis have not been reported. We aimed to define the duration of disease, number of treatment lines, treatment course, extramedullary plasma cell tumor, hematopoietic stem cell transplantation, FISH abnormal gene number, risk (combined FISH and second-generation sequencing analysis) ,feasibility, toxicity, maximum tolerated dose, response rate, and biological correlates of response in patients with malignant plasma cell disease treated with BCMA-CAR T cells. This trial is registered with ChiCTR, number ChiCTR-OPC-16009113.
Methods:
Between March 10, 2017, and March 27, 2018, 28 patients (including 26 patients with relapsed or refractory MM, 1 patient with Plasma cell leukemia and 1 patient with POEMS) were enrolled and infused with BCMA-CAR T cells. The CAR-BCMA chimeric antigen receptor was encoded by the lentiviral vector and contained a murine anti-BCMA single-chain variable fragment, a CD8a hinge, the CD28 transmembrane regions and intracellular domain and CD3-ζ T-cell activation domain. Peripheral blood mononuclear cells were collected from the patient by leukapheresis, and whole peripheral blood mononuclear cells were cultured and transduced. Patients received a target dose of 5.4 ~ 25.0×106 anti-BCMA CAR T cells per kilogram of body weight after a conditioning chemotherapy regimen of cyclophosphamide and fludarabine. MM response assessment was conducted according to the International Uniform Response Criteria for MM. Cytokine-release syndrome (CRS) was graded as Lee DW et al. described (Current concepts in the diagnosis and management of cytokine release syndrome. Blood. 2014;124(2):188-195.)
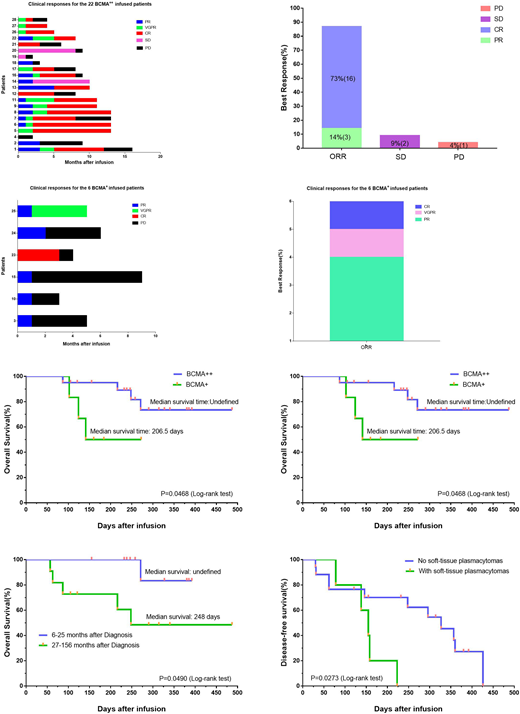
Results: Twenty-six of 28 treated patients obtained remission. According to the expression rate of BCMA on the surface of plasma cells, all patients were divided into BCMA strong expression group (22 cases, BCMA expression rate ≥ 50%) and BCMA weak expression group (6 cases, BCMA expression rate <50%). The overall response rate for BCMA strong expression group was 87%, with 73% complete response. The overall response rate for the BCMA weak expression group was 100%, with 33% very good partial response or complete response (Figure a-d). The overall survival of the two groups was undefined (a strong group) and 206.5 days (a weak group) respectively (P=0.0468). The median disease-free survival of the two groups was 296 days (strong group, 20 responded cases) and 64 days (weak group,6 cases) respectively (P=0.0069) (Figure e, f). Patients with longer duration have shorter overall survival time. Patients with soft-tissue plasmacytomas have short disease-free survival (155 days vs. 327 days) (Figure g, h). All toxicities were fully reversible, with the most severe being grade 3 cytokine release syndrome that occurred in four of 28 patients. High peak blood CAR+ cell levels were associated with anti-MM responses. Blood CAR-BCMA T cells were predominantly highly differentiated CD8+ T cells after infusion.
Conclusions: BCMA-CAR T cell therapy is feasible, safe, and mediates potent anti-tumor activity in relapsed/refractory Multiple Myeloma. All toxicities were reversible Our results should encourage additional development of CAR T-cell therapies for other plasma cell malignancies such as plasma cell leukemia and POEMS.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.